Do you know someone with Hodgkin’s Lymphoma? Researchers made an incredible connection between this lymphatic cancer and the immune system.
Cancer research is one of the most important research areas of the century. The cases of cancer that people face each year are innumerable, yet the disease and the cure is elusive. It’s like an enemy that has the perfect hiding spot and attacks when you least expect them to.
The fight against cancer is raging on, and it seems that researchers have some victorious battles lately. One of these victories was by scientists at The Institute of Cancer Research, London. They’ve identified a genetic link between the immune system and Hodgkin’s lymphoma. Keep reading for the details of the study.
What is Hodgkin’s Lymphoma?
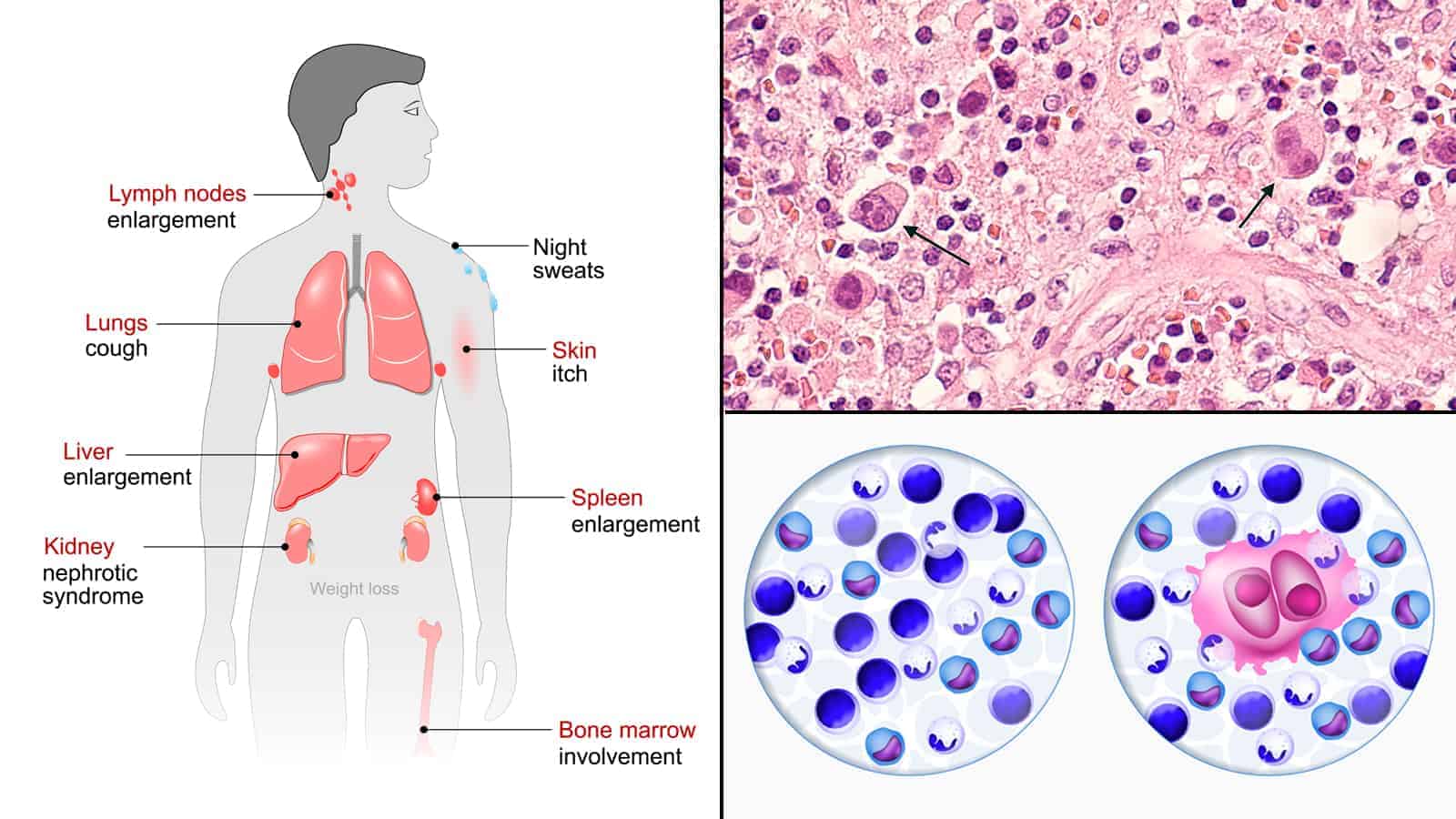
To better understand the research, it may help if you know what Hodgkin’s lymphoma is. It’s is one of two types of lymphoma cancers. The second type is non-Hodgkin’s lymphoma. They’re both rare cancers, with Hodgkin’s being even more infrequent than non-Hodgkin’s.
There are only two slight difference between Hodgkin’s and non-Hodgkin’s. If the cancerous cells contain Reed-Sternberg cells, then it’s classified as Hodgkin’s. If not, it’s classified as non-Hodgkin’s. The second difference is that Hodgkin’s is a little more predictable than non-Hodgkin’s so it’s easier to detect early, leading to more successful treatments.
The research specifically focuses on Hodgkin’s lymphoma. Hodgkin’s can be further classified into two subtypes. Scientists designate them as classical Hodgkin’s lymphoma (cHL) and nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL). This research focuses on cHL which makes sense because it accounts for 95% of Hodgkin’s lymphoma cases.
If that’s not confusing enough, cHL can be further classified into four sub-subtypes:
- Nodular sclerosis (NSHL)
- Mixed cellularity (MCHL)
- Lymphocyte-rich
- Lymphocyte-depleted
The research focuses on the first two. NSHL comprises about 60% of cHL cases while MCHL comprises about 20%.
The Lymph System
As you may know, cancer starts anywhere in the body where cells begin to grow out of control. Lymphoma cancer is, as the name suggests, cancer of the lymph system.
The lymph system is a part of the immune system. It’s composed of vessels that are a lot like blood vessels. However, instead of carrying blood, they carry a watery, clear fluid called lymph (hence the name of the system).
This fluid is responsible for removing waste, bacteria, and viruses. Think of the lymph system as giving a bath to your tissues. The vessels carry lymph around your body, and the fluid filters through the hundreds of lymph nodes throughout your body.
How Cancer Affects the Lymph System
There are two ways that the lymph system develops cancer. The cancer could start in the lymph nodes or the cancer could start somewhere else, break off (called metastasis), and travel to the lymph nodes. According to the American Cancer Society, the latter method occurs more than the former.
When lymph nodes are infected with cancer (or other foreign invaders), they swell up due to a lymphatic obstruction. This simply means that lymph is unable to filter through the node. Sometimes they can swell up big enough for you to feel or even see under the skin. The swelling of lymph nodes is known as lymphedema.
Lymphoma cancer invades lymphocytes found in white blood cells. There are two types of lymphocytes: B cells and T cells. Hodgkin’s lymphoma usually affects B cells first.
Where Lymphoma Can Occur in the Body
Like all cancers, lymphoma can occur mostly anywhere in the human body. That’s because the lymph system spreads throughout the body. The most common areas are:
- Spleen
- Thymus
- Bone Marrow
- Adenoids/tonsils
- Digestive tract
Lymphoma mostly starts in the top half of the body but it’s not uncommon for it to occur in the lower half.
Now that you’re up to speed with the cancer, you’ll be able to follow along with the research more efficiently. We will unpack all the research in greater detail in the next section.
Details of the Research
The research and its subsequent breakthrough were made by the Cancer Genomics Team from the Institute of Cancer Research, London (ICR). The team is lead by Professor Richard Houlston, Head of the Division of Genetics and Epidemiology. Other members of the group included:
- Dr. Amit Sud, an Academic Clinical Lecturer in Genetics and Epidemiology at ICR
- Dr. Hauke Thomsen, Senior Bioinformatician and Biostatistician
- Dr. Kari Hemminki of the Division of Molecular Genetic Epidemiology at the German Cancer Research Centre
There were many other researchers involved, but these four led and supervised the team. The research was first published in the Nature Communications journal on December of 2017.
Introduction of the Research
While cHL is rare and, in most cases, successfully treated. Still, scientists continue to research how to improve treatments, especially for people in which first-line treatments don’t work. Past research has shown a well-established connection between the human leukocyte antigen (HLA) region and cHL. However, there hasn’t been much research into how genetics plays into the development of the cancer.
This research project was started to identify heritable risk factors for cHL, which are largely unknown. The initial theory of inherited genetic influence of cHL came from a trend of cHL running in families. However, it was strongly influenced by cases of identical twins both developing cHL.
Researchers believed that this study would be widely successful because of recent studies composed of data from the genome-wide association studies (GWAS). The next section will give you some information about GWAS.
The team used data from the The data came from three different studies in the GWAS; one was a new study from the UK National Study of Hodgkin Lymphoma Genetics (NSHLG) and the other two from two pre-existing studies in the GWAS database.
The subjects of their data was of European ancestry which seems a bit limited at first glance. However, the cancer disproportionately affects people of European origin, so this is a good pool of data to use. In all, scientists analyzed data from over 22,000 subjects. This was one of the largest research studies for cHL ever done.
A Brief Description of GWAS
GWAS is essentially a database of research, studies, statistics, and other information that researchers can pull data from. The purpose of this database is to have a centralized location from which researchers all over the world can pull or pool data. This leads to a much more efficient way of researching any genome-related project.
GWAS was started with the completion of the Human Genome Project. This was one of the biggest successes in the history of modern science. Researchers set out to map every single gene in the human body (collectively called the human genome or just genome). The project took 13 years to complete, lasting from 1990 to 2003.
The project was an international effort. Their team included researchers and scientists from the United States, France, Germany, China, the United Kingdom, and Japan. By creating a blueprint of the DNA sequencing of the entire human body this project advanced science exponentially.
The project and decades of research has turned into a publicly assessable database for scientists to use. Pooling data from all over the world makes it much easier to conduct research without needing to set up individual projects, which could take years. The data is already there – they just need to analyze it and look for trends.
GWAS and the Human Genome Project has been more successful than anyone could have hoped for. It has even led to an era of personalized medicines and treatments. The database continues to grow with every successful research project that stems from it.
The Results of the Study
After analyzing the data, researchers discovered a link between genetic mutations and cHL. In fact, they identified six new single-nucleotide polymorphisms (SNPs) at non-HLA risk locations. SNPs are variations of a single position in a DNA sequence. For science to consider this an SNP, over 1% of the population must have a variation at this position.
These SNPs cause transcription factor binding, essentially meaning they can connect to and possibly disable adjacent genes. The significance of this is that five of these six newly identified SNPs are next to cells that have been previously associated with the development of cHL or B-cells. This leads scientists to the conclusion that these SNPs are strong risk factors for cHL.
Final Thoughts on the Study and Hodgkin’s Lymphoma
The GWAS has been a catalyst for a slew of new research and etiology. This study is a great example of this. It provided a new, important insight into the development of cHL which could be used in the future to detect people of high risk and possible even provide them with preventative treatment.
Cancer research is incredibly important in these times, so any research like this is welcomed in the medical field. Hopefully, the GWAS will continue to fuel new developments that will make life better for everyone, especially those battling cancer. The future of human health depends on it.